Ich Validation Of Analytical Procedures
Whether you’re organizing your day, mapping out ideas, or just want a clean page to jot down thoughts, blank templates are super handy. They're simple, versatile, and easy to customize for any use.
Stay Flexible with Ich Validation Of Analytical Procedures
These templates are perfect for anyone who likes a balance of structure and freedom. You can use unlimited copies and fill them out by hand, making them ideal for both personal and professional use.

Ich Validation Of Analytical Procedures
From graph pages and ruled paper to checklists and planners, there’s plenty of variety. Best of all, they’re easy to download and printable from your own printer—no signup or extra tools needed.
Free printable blank templates keep things tidy without adding complexity. Just pick what fits your needs, grab some copies, and start using them right away.
2005 ICH Q2 R1 Validation Of Analytical Procedures Text And
Jun 5 2025 nbsp 0183 32 ICH M4Q R2 Nov 27, 2024 · ICH Q1B规定的样品应暴露在总照度不低于1.2×106Lux·hr,近紫外能量不低于200(w·hr)/m2,这个数值与货架期之间有什么联系吗?

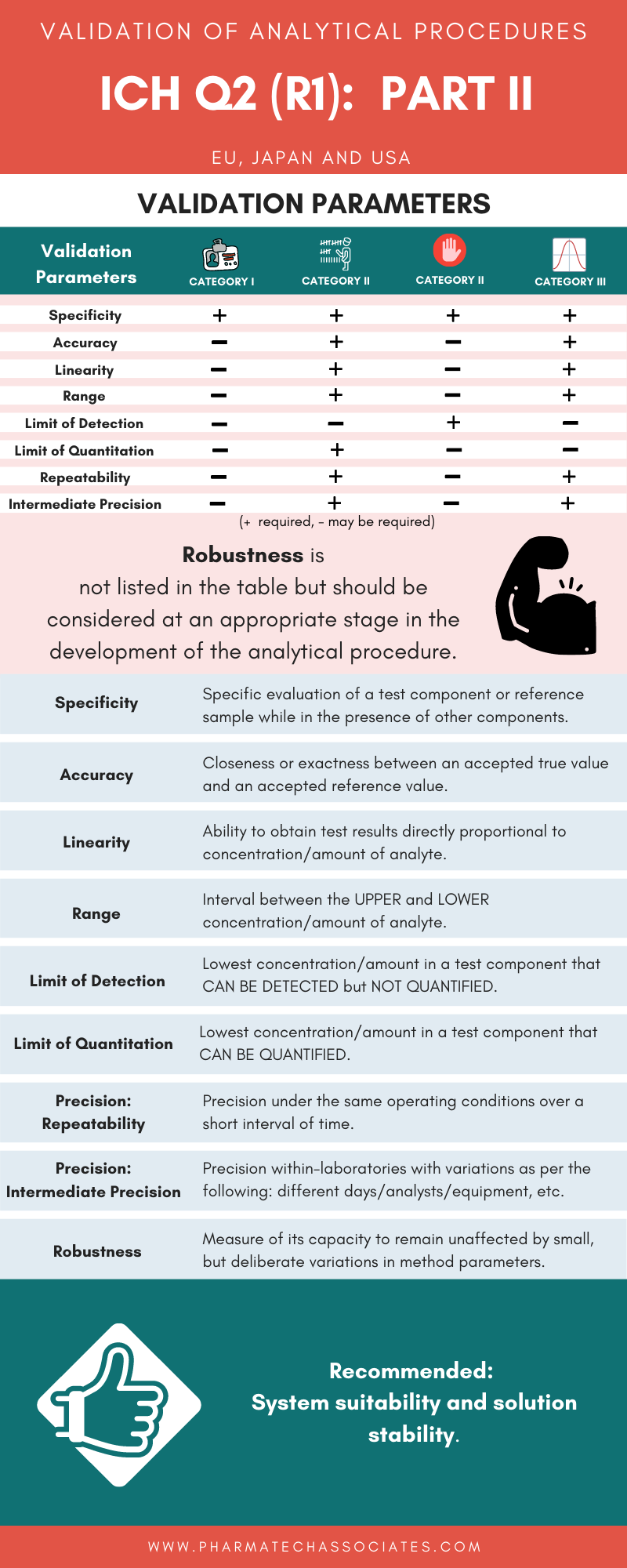
Validation Parameters Of Analytical Methods As Per ICH Guidelines PART
Ich Validation Of Analytical ProceduresMar 18, 2020 · 中国2017年正式加入ICH,CDE在陆续发布ICH指南及其中文译稿,也在要求制药行业参考ICH指南执行。 为方便学习使用,本人整理下载了目前CDE官方发布出来的ICH指南, … May 18 2025 nbsp 0183 32 PMI ICH M7
Gallery for Ich Validation Of Analytical Procedures

International Council For Harmonization ICH History Purpose Body

Performance Characteristic Validation Of Analytical Procedures As Per

General Considerations For Validation Of Analytical Procedures As Per
Controle Technique Ctm 31 Safe Shipping Www gbu presnenskij ru
![]()
Parallel Session 6 ICH Q14 And Q2 R2 Concepts And Enhanced
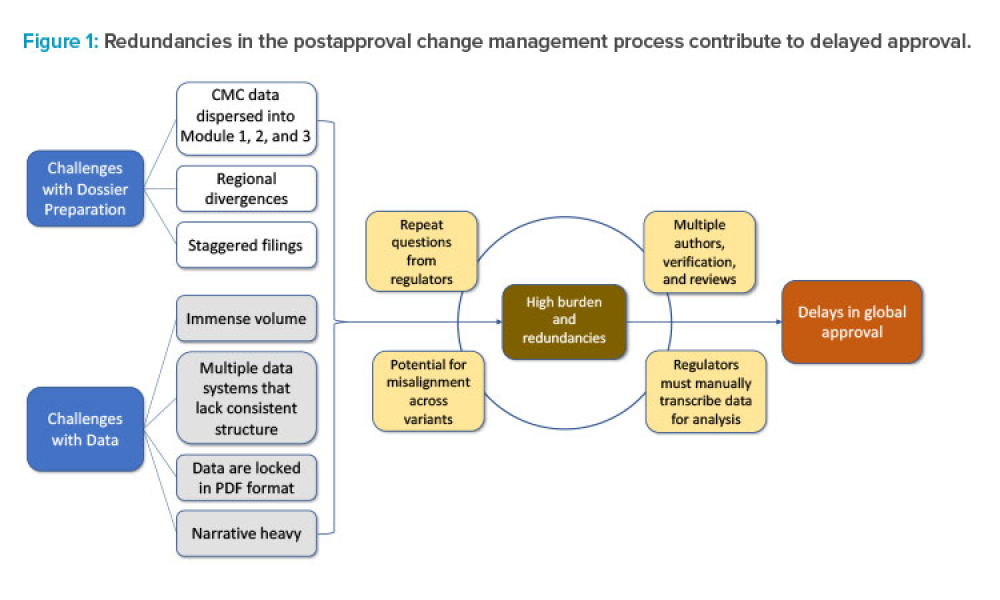
Regulatory ISPE International Society For Pharmaceutical Engineering
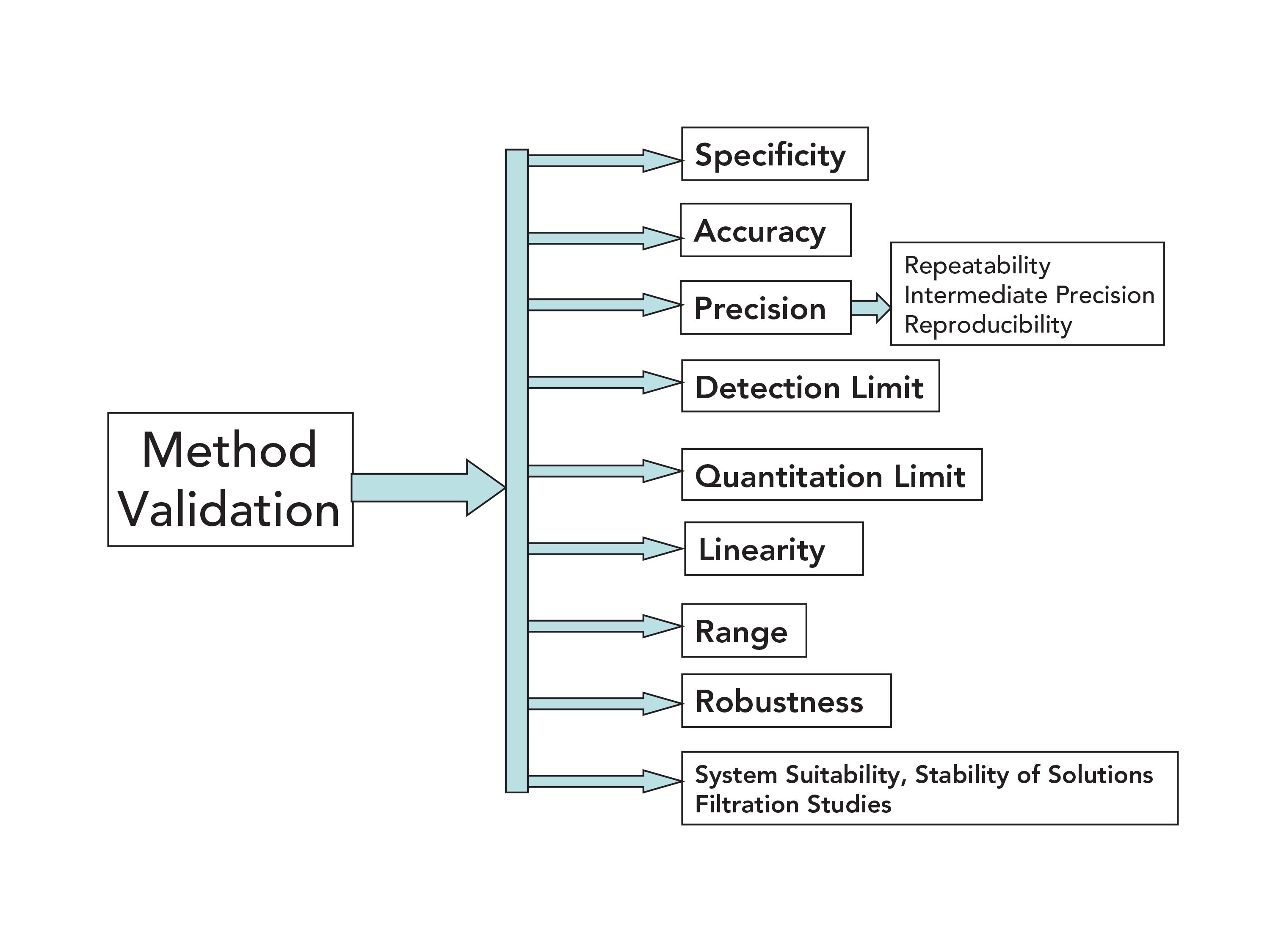
Analytical Method Validation Pharma Compliance Part 2

ICH Q2 Q14 Analytical Procedure Life Cycle Management Chromicent

Analytical Method Validation Its Role In Lab Testing GMP Insiders

ICH Q2 R2 The Latest ICH Guideline For Analytical Procedures Validation
