Ich Q2 Analytical Method Validation
Whether you’re setting up your schedule, mapping out ideas, or just want a clean page to jot down thoughts, blank templates are incredibly helpful. They're clean, practical, and easy to adapt for any use.
Stay Flexible with Ich Q2 Analytical Method Validation
These templates are perfect for anyone who likes a balance of structure and freedom. You can print as many as you like and fill them out by hand, making them great for both personal and professional use.

Ich Q2 Analytical Method Validation
From graph pages and ruled paper to to-do formats and planners, there’s something for everyone. Best of all, they’re easy to download and printable at home—no registration or extra tools needed.
Free printable blank templates help you stay organized without adding complexity. Just pick what fits your needs, grab some copies, and start using them right away.

General Considerations For Validation Of Analytical Procedures As Per
Jun 24 2022 nbsp 0183 32 European Medicines Agency E Jun 5, 2025 · 蒲公英 - 制药技术的传播者 GMP理论的实践者 » 蒲公英论坛 › 研发&生产 › 药品研发 › ICH人用药品注册通用技术文件M4Q(R2)
![]()
Parallel Session 6 ICH Q14 And Q2 R2 Concepts And Enhanced
Ich Q2 Analytical Method ValidationFeb 20, 2023 · 摘要起始物料的选择及质量控制是原料药药学研究的重要组成部分,而起始物料的选择问题一直备受关注。本文对 ICH,EMA,FDA 及我国起始物料的最新要求进行了综述, … Apr 16 2014 nbsp 0183 32 ICH HARMONISED TRIPARTITE GUIDELINE JuliaSTABILITY TESTING PHOTOSTABILITY TESTING OF NEW DRUG SUBSTANCES AND PRODUCTS
Gallery for Ich Q2 Analytical Method Validation
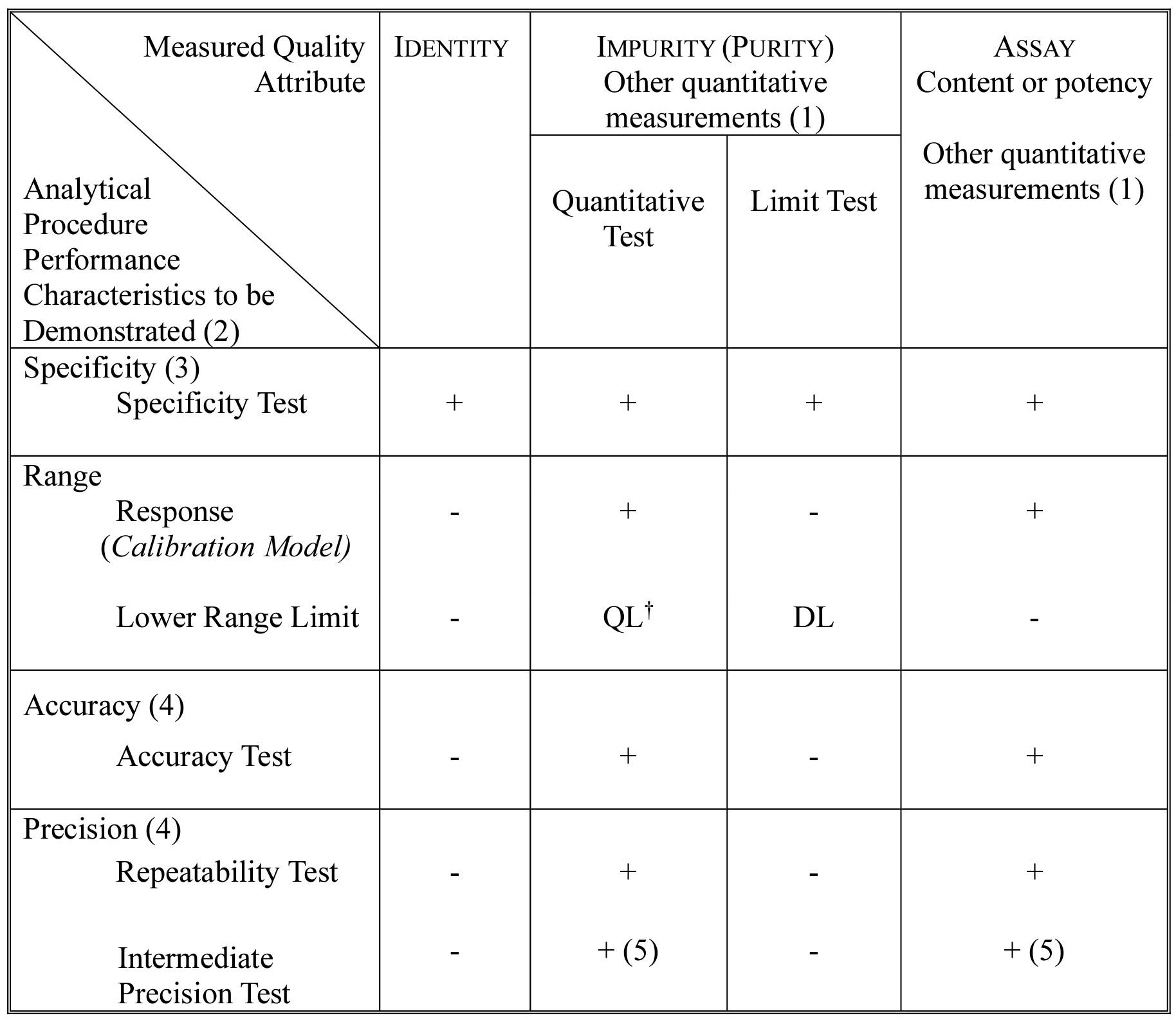
New Update For The ICH Q2 R2 Guidance On Analytical Process

ICH Q2 Q14 Analytical Procedure Life Cycle Management Chromicent

Analytical Method Validation Its Role In Lab Testing GMP Insiders

Understanding ICH Q2 R2 Q14 Updates On Robustness Studies

ICH Guideline Q2 Analytical Validation Pharmabeej

Performance Characteristics In Analytical Method Validation

ICH Q2 R2 Guideline On Validation Of Analytical Procedures Step 5
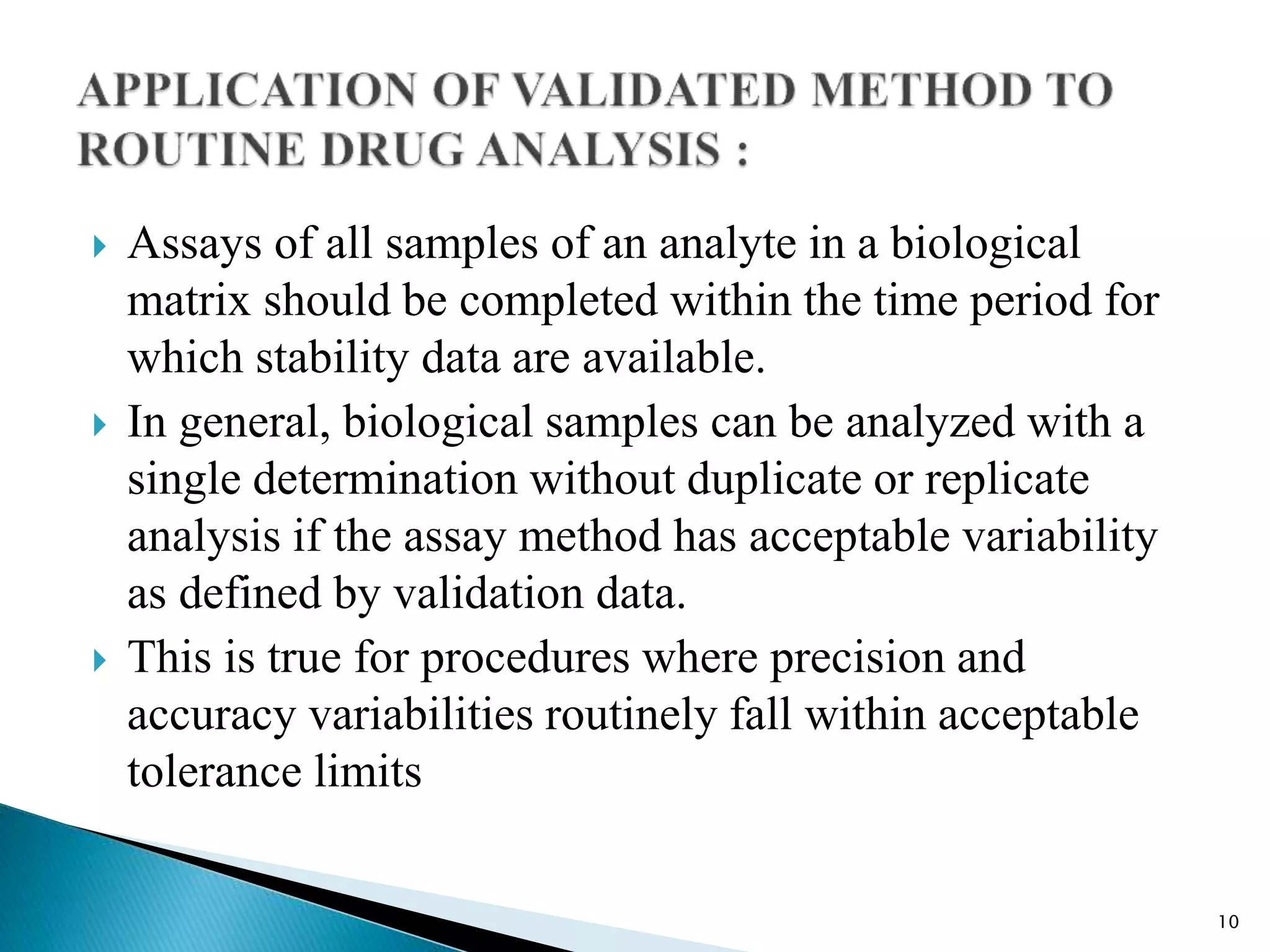
M10 Bioanalytical Method Validation PPTX
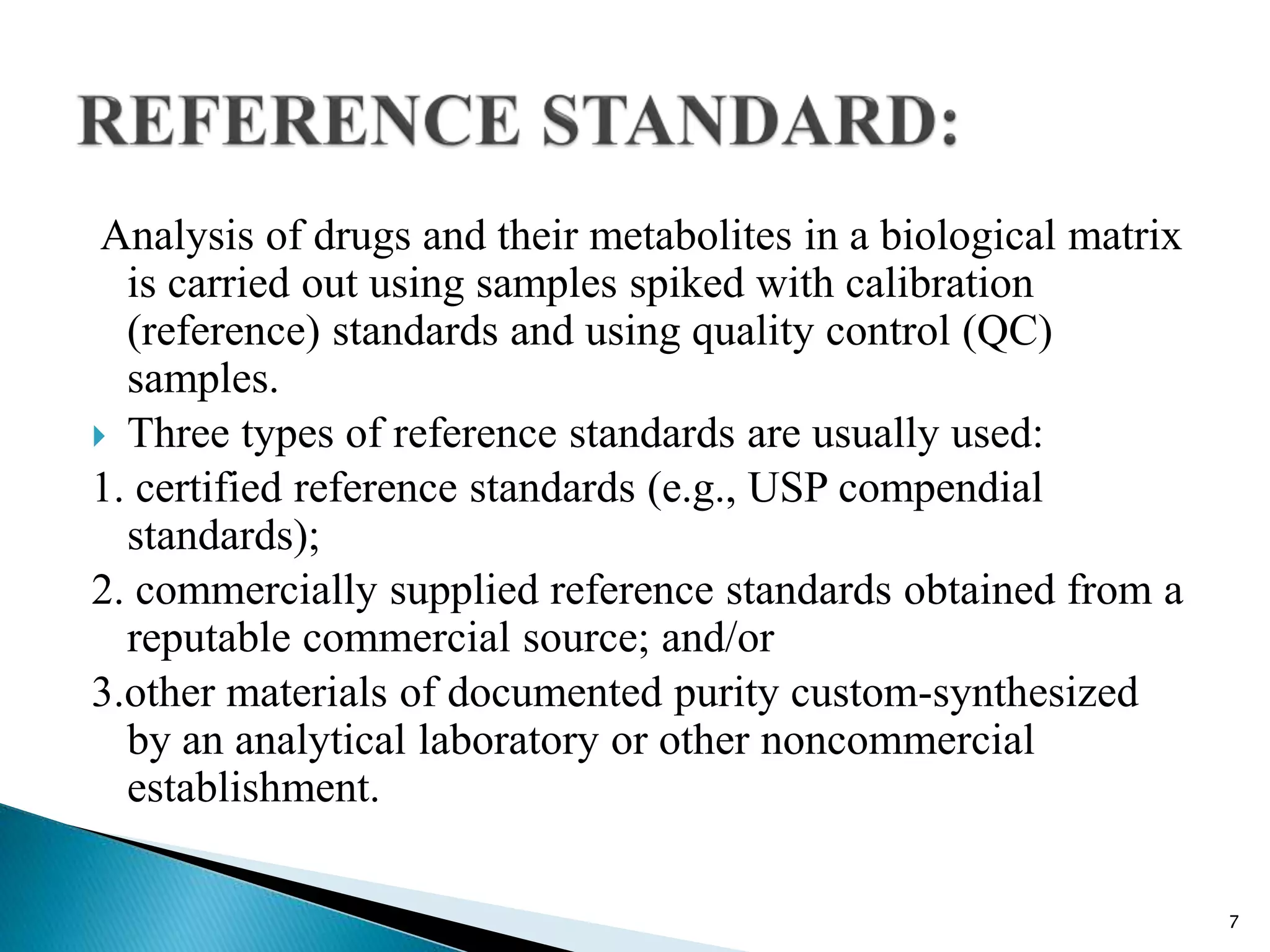
M10 Bioanalytical Method Validation PPTX

M10 Bioanalytical Method Validation PPTX